|
Forecast Period
|
2027-2031
|
|
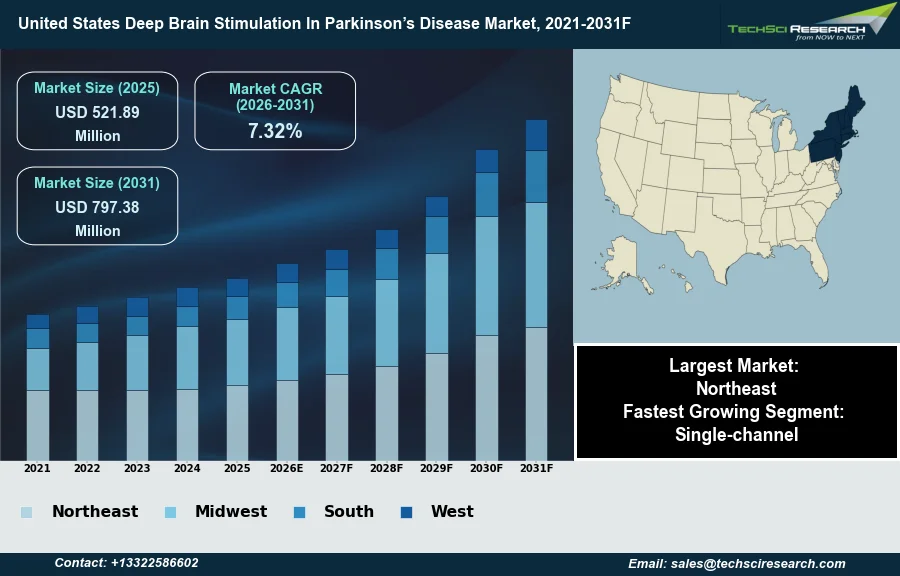
Market Size (2025)
|
USD 521.89 Million
|
|
CAGR (2026-2031)
|
7.32%
|
|
Fastest Growing Segment
|
Single-channel
|
|
Largest Market
|
Northeast
|
|
Market Size (2031)
|
USD 797.38 Million
|
Market Overview
The United States Deep Brain Stimulation In Parkinson’s Disease Market will grow from USD 521.89 Million in 2025 to USD 797.38 Million by 2031 at a 7.32% CAGR. Deep Brain Stimulation (DBS) for Parkinson’s disease involves the surgical implantation of a pulse generator and electrodes that deliver precise electrical signals to targeted brain structures, such as the subthalamic nucleus, to modulate abnormal neural activity and alleviate motor symptoms. The market in the United States is primarily driven by an expanding geriatric demographic and the consequent rise in neurodegenerative diagnoses, creating a critical demand for therapeutic interventions that offer sustained efficacy when pharmacotherapy becomes insufficient. According to the Parkinson’s Foundation, in 2025, the combined economic burden of Parkinson’s disease in the United States was estimated to be nearly $61.5 billion, underscoring the immense scale of the condition and the urgent market need for cost-effective, long-term management solutions.
However, market expansion faces a significant impediment regarding the high costs associated with the device and the implantation procedure. The substantial financial investment required for the surgery and post-operative care, coupled with complex reimbursement and coverage limitations, restricts patient accessibility and serves as a considerable barrier to the widespread adoption of this neurosurgical therapy.
Key Market Drivers
Technological evolution of directional leads and closed-loop stimulation systems is propelling the United States Deep Brain Stimulation (DBS) market by directly addressing the clinical need for precision and adaptability in neurosurgical interventions. The shift toward sensing-enabled devices allows physicians to visualize patient-specific brain signals and steer electrical stimulation to targeted neural structures, significantly reducing off-target side effects compared to conventional omnidirectional leads. This innovation cycle is translating into substantial commercial gains for manufacturers that have successfully commercialized these next-generation platforms. According to Abbott, April 2024, in the 'First-Quarter 2024 Results', the company’s Neuromodulation division recorded an organic sales growth of 17.4%, a surge driven largely by the strong market uptake of its advanced Eterna and Liberta implantable systems. Such robust growth metrics indicate a decisive market preference for miniaturized, rechargeable devices that integrate remote programming capabilities, thereby enhancing long-term patient management.
Escalating prevalence of Parkinson’s disease amidst an aging US population serves as the fundamental engine for market volume, creating a widening gap between the number of eligible patients and those currently receiving therapy. As the demographic profile of the country shifts, the incidence of motor disorders is accelerating, necessitating scalable therapeutic alternatives to pharmacotherapy. According to the American Parkinson Disease Association, September 2024, in the 'How many people in the US have Parkinson's disease?' article, there are approximately 90,000 new diagnoses of the condition in the United States annually. This rising incidence rate underscores the critical urgency for expanding access to neurostimulation procedures. Reflecting the scale of this demand, according to Boston Scientific, in 2024, the Neuromodulation business segment generated $256 million in net sales during the first quarter alone, highlighting the significant economic value currently actively managed within this therapeutic sector.
Download Free Sample Report
Key Market Challenges
The prohibitive costs associated with the device and the implantation procedure constitute a primary restraint on the growth of the United States Deep Brain Stimulation in Parkinson’s Disease Market. The substantial capital required for the neurostimulator and the complexity of the neurosurgical intervention create a high barrier to entry for many healthcare providers. Hospitals often face significant financial risks due to the disparity between the high procurement costs of the technology and the rigid, often insufficient, payment rates offered by insurance providers, which discourages the widespread expansion of deep brain stimulation programs.
Furthermore, this financial pressure is exacerbated by a complex reimbursement landscape that enforces strict eligibility criteria, thereby limiting patient access. The economic burden of managing the condition is already immense, making payers hesitant to approve high-cost surgical therapies without exhaustive prerequisites. According to the International Parkinson and Movement Disorder Society, in 2024, the average annual healthcare cost for a patient with Parkinson’s disease was estimated to be approximately $23,200, a baseline expenditure that surges dramatically when adding the upfront costs of surgical implantation. This compounding financial weight compels insurers to maintain restrictive coverage policies, directly impeding the market’s ability to reach a broader patient population.
Key Market Trends
The shift toward robotic-assisted neurosurgical implantations is fundamentally altering the procedural landscape of the United States Deep Brain Stimulation (DBS) market by establishing new standards for surgical precision and safety. Unlike traditional frame-based stereotactic methods, robotic systems minimize human error and streamline the operative workflow, which is crucial for placing electrodes into minute brain targets such as the subthalamic nucleus. This technological integration not only enhances surgeon confidence but also improves clinical outcomes by ensuring consistent, high-accuracy lead placement. According to the National Institutes of Health, May 2024, in the 'Efficacy and safety of robot-assisted deep brain stimulation for Parkinson’s disease' article, a meta-analysis of the technology revealed that the pooled vector error for robot-assisted lead placement was measured at just 1.09 mm, highlighting the exceptional accuracy driving the adoption of these automated platforms in American hospitals.
Concurrently, the application of artificial intelligence for personalized therapy tuning is revolutionizing post-operative patient management by moving beyond static stimulation parameters. Innovative adaptive DBS systems now utilize AI algorithms to continuously interpret neural biomarkers and dynamically adjust electrical delivery in real-time, responding instantly to fluctuations in patient symptoms. This automation addresses a critical gap in conventional therapy, where fixed settings often fail to account for the variable nature of motor deficits throughout the day. According to the Parkinson’s Foundation, October 2024, in the 'New Study Further Personalizes Deep Brain Stimulation' article, clinical trials utilizing these AI-driven adaptive systems demonstrated that participants experienced nearly a 50% reduction in time spent with their most troublesome symptom compared to conventional stimulation, validating the market's trajectory toward intelligent, data-driven neuromodulation.
Segmental Insights
Market analysis reveals that the Single-channel segment is currently the fastest-growing category within the United States Deep Brain Stimulation In Parkinson’s Disease Market. This trend is principally driven by the economic advantages of single-channel neurostimulators, which provide a cost-effective treatment option, thereby expanding patient access relative to multi-channel alternatives. Moreover, neurologists increasingly utilize these devices for their specific programming versatility, which proves advantageous for managing distinct motor symptoms. These drivers, combined with the device's suitability for unilateral lead implantation, are fueling its accelerated adoption across the United States healthcare landscape.
Regional Insights
The North-East region maintains a leading position in the United States Deep Brain Stimulation in Parkinson’s Disease market due to a dense concentration of specialized medical centers and academic research institutions. This region benefits significantly from the headquarters of major industry players, such as Boston Scientific in Massachusetts, which facilitates established distribution networks and clinical collaboration. Additionally, high healthcare spending and favorable reimbursement policies in states like New York encourage the adoption of these medical devices. The proximity of these facilities to a large patient base ensures consistent market stability for these therapeutic interventions.
Recent Developments
-
In December 2025, Boston Scientific introduced new directional leads for its deep brain stimulation portfolio, specifically designed to deliver more precise symptom relief for individuals with Parkinson's disease. The new Vercise Cartesia X and Vercise Cartesia HX leads were developed to be used with image-guided programming, enabling neurologists to target specific brain areas more accurately while minimizing potential side effects. The company highlighted that these leads offered a significantly greater directional span than previous models, allowing for enhanced flexibility in stimulating multiple sites to address complex symptoms such as tremor and gait issues.
-
In February 2025, Medtronic announced the U.S. Food and Drug Administration approval of its BrainSense Adaptive deep brain stimulation system for patients with Parkinson's disease. This approval introduced a closed-loop therapy that automatically adjusted stimulation levels in real-time based on the patient's detected brain signals, aiming to provide a more personalized treatment experience. The system was designed to manage abnormal brain activity associated with involuntary muscle movements and minimize the need for manual adjustments by physicians. The launch was supported by data from a large-scale trial demonstrating the safety and efficacy of the adaptive algorithm compared to continuous stimulation.
-
In August 2024, Medtronic received approval from the U.S. Food and Drug Administration for its Asleep Deep Brain Stimulation surgery for the treatment of Parkinson's disease and essential tremor. This regulatory decision established the company as the first to offer a deep brain stimulation solution approved for use while a patient is either asleep under general anesthesia or awake. The approval provided an alternative for patients who might be apprehensive about awake surgery, with clinical data indicating that the asleep procedure maintained patient safety and offered comparable improvement in motor symptoms to traditional awake methods.
-
In January 2024, Abbott announced that it received approval from the U.S. Food and Drug Administration to launch the Liberta RC deep brain stimulation system. This device was introduced as the smallest rechargeable deep brain stimulation implant with remote programming capabilities for treating movement disorders, including Parkinson's disease. The system was designed to require fewer recharge sessions than other available technologies, needing approximately ten charges per year under standard settings. The approval also enabled compatibility with the company's virtual clinic platform, allowing patients to communicate with healthcare providers and receive therapy adjustments remotely without visiting a clinic.
Key Market Players
- Boston Scientific Corporation
- Abbott Laboratories Inc
- Medtronic plc
- Functional Neuromodulation Inc
- Nuvectra Corp
- Aleva Neurotherapeutics SA
|
By Product
|
By Region
|
- Single-channel
- Dual-channel
|
- Northeast
- Midwest
- South
- West
|
Report Scope:
In this report, the United States Deep Brain Stimulation In Parkinson’s Disease Market has been segmented into the following categories, in addition to the industry trends which have also been detailed below:
-
United States Deep Brain Stimulation In Parkinson’s Disease Market, By Product:
-
Single-channel
-
Dual-channel
-
United States Deep Brain Stimulation In Parkinson’s Disease Market, By Region:
-
Northeast
-
Midwest
-
South
-
West
Competitive Landscape
Company Profiles: Detailed analysis of the major companies present in the United States Deep Brain Stimulation In Parkinson’s Disease Market.
Available Customizations:
United States Deep Brain Stimulation In Parkinson’s Disease Market report with the given market data, TechSci Research offers customizations according to a company's specific needs. The following customization options are available for the report:
Company Information
- Detailed analysis and profiling of additional market players (up to five).
United States Deep Brain Stimulation In Parkinson’s Disease Market is an upcoming report to be released soon. If you wish an early delivery of this report or want to confirm the date of release, please contact us at [email protected]