|
Forecast Period
|
2026-2030
|
|
Market Size (2024)
|
USD
79.09 Million
|
|
CAGR (2025-2030)
|
10.37%
|
|
Fastest Growing Segment
|
Small
Molecule
|
|
Largest Market
|
South India
|
|
Market Size (2030)
|
USD 143.28 Million
|
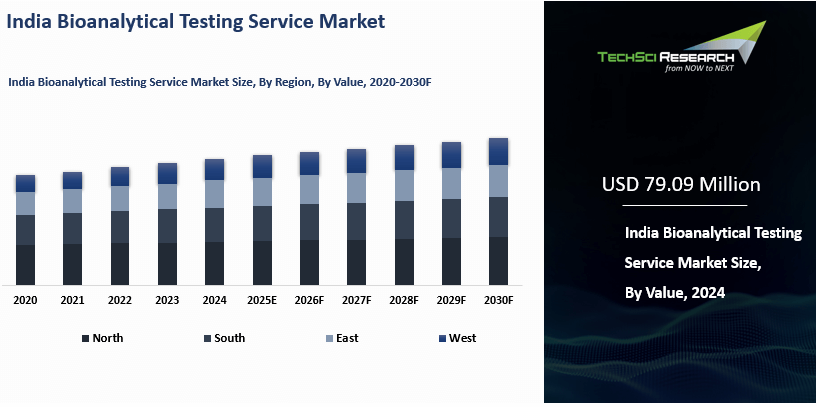
Market Overview
India Bioanalytical Testing Service
Market was valued at USD 79.09 Million in 2024 and is expected to reach USD 143.28
Million by 2030 with a CAGR of 10.37% during the forecast period.
The India
Bioanalytical Testing Service market is experiencing robust growth, primarily
driven by the increasing demand from the pharmaceutical and biotechnology
sectors. The rising need for drug efficacy, safety, and regulatory compliance
has led to a surge in bioanalytical testing services. Outsourcing of these
services to India, driven by cost-effectiveness and a skilled talent pool, has
further fueled market expansion. The growing number of clinical trials and
stringent regulatory requirements by bodies like the CDSCO and WHO have also
contributed to the rising demand for bioanalytical testing. Additionally,
advancements in technologies such as mass spectrometry and chromatography have
enhanced testing capabilities, attracting more companies to utilize these
services.
However, the market faces challenges such as the
complexity of testing for biologics, which requires specialized equipment and
expertise. The lengthy regulatory approval processes and high costs associated
with setting up advanced testing laboratories can also be hurdles. Furthermore,
there is an increasing need for skilled professionals to meet the growing
demand, which may create talent shortages in the future. Despite these
challenges, the India Bioanalytical Testing Service market is poised for continued
growth, with the Southern region emerging as the dominant player, benefiting
from the concentration of pharmaceutical and biotech firms, strong government
support, and an established infrastructure.
Download Free Sample Report
Key Market Drivers
Rising
Demand for Pharmaceutical and Biotech Research
The rising demand for pharmaceutical and biotech research in India is fueled by multiple factors that align with global healthcare trends. India is a significant player in the clinical trials landscape, conducting about 8% of global clinical trials as of 2022. This is supported by the country's large and diverse patient pool of over 1.4 billion people, which provides valuable demographic data for clinical research. India's cost advantages, with trials being significantly cheaper than in Western nations, and a skilled, English-speaking medical workforce further enhance its appeal as a hub for all phases of clinical trials.
India’s pharmaceutical sector plays a crucial role in the global supply chain. The country is the largest provider of generic medicines globally, accounting for a 20% share of the global supply by volume. It supplies approximately 60% of the world's vaccine demand and fulfills 65-70% of the World Health Organization's vaccine requirements. In the fiscal year 2024, India's pharmaceutical exports reached $27.8 billion. Consequently, both domestic and international pharmaceutical companies are increasing their R&D spending in India, which necessitates advanced bioanalytical testing services to ensure the safety and efficacy of new drugs.
The Indian government actively supports this growth through various initiatives.
- The Production Linked Incentive (PLI) schemes for pharmaceuticals, bulk drugs, and medical devices have a total financial outlay of over ₹25,000 crore (approximately $3 billion USD). As of late 2024 and early 2025, these schemes had already attracted investments significantly higher than the initially committed amounts
- The scheme aims to attract a total R&D investment of approximately ₹11,000 crore, not ₹17,000 crore. This investment will be used to support around 300 projects involving new medicines, complex generics, biosimilars, and novel medical devices. Some sources incorrectly stated the higher figure, likely conflating targets or referring to outdated information.
- The Biotechnology Industry Research Assistance Council (BIRAC) provides funding through programs like the Biotechnology Ignition Grant (BIG), which offers up to ₹50 lakhs (around $60,000) to support early-stage research projects with commercial potential.
India's growing role in biotechnology innovation is also evident from its significant contributions to vaccine development. The country has the highest number of US FDA-compliant pharmaceutical plants outside of the United States. It is a leading producer of affordable vaccines for diseases such as measles, DPT (Diphtheria, Tetanus, and Pertussis), and polio. For instance, India accounts for nearly 90% of the global demand for the measles vaccine. During the COVID-19 pandemic, India supplied 242 million low-cost, high-quality vaccine doses to 101 countries. This focus on biotechnology, along with advancements in genomics and personalized medicine, is increasing the demand for bioanalytical testing services as companies require these services to meet stringent regulatory standards.
Surge in Technological
Advancements
The increase in clinical trials in India
has been a significant driver of the rising demand for pharmaceutical and
biotech research, along with bioanalytical testing services. India has emerged
as a preferred destination for clinical trials due to its large, diverse
population, cost-effectiveness, and efficient regulatory processes. As of
recent reports, India conducts over 1,000 clinical trials annually, with a
substantial proportion—approximately 60-70%—being sponsored by international
pharmaceutical companies. This makes India one of the leading locations
globally for clinical trial activities.
The country’s vast patient pool, which
exceeds 1.4 billion people, is a major attraction for pharmaceutical companies.
India's population includes a broad spectrum of genetic backgrounds, providing
valuable insights into the effectiveness and safety of new drugs across diverse
demographic groups. This is particularly beneficial for trials related to
chronic conditions such as diabetes, hypertension, cancer, and cardiovascular
diseases, which are prevalent in India. For example, global pharmaceutical companies
like Novartis and Pfizer have conducted clinical trials in India for drugs
targeting diabetes and oncology, benefiting from the country’s large patient
base and cost advantages.
India’s clinical trials also offer
significant cost savings compared to developed nations. The cost of
conducting clinical trials in India is often 40-60% lower than in countries
like the United States or Europe. This includes reduced costs for patient
recruitment, data collection, and clinical monitoring. A study by the Clinical
Trials Registry of India revealed that clinical trial costs in India are
approximately one-fourth of those in the U.S., making it an attractive option
for global drug developers.
India’s regulatory framework, overseen
by the Central Drugs Standard Control Organization (CDSCO), has evolved in
recent years to support the growing clinical trials market. The government has
introduced reforms to expedite the approval process for new trials and has
worked to align regulatory practices with global standards such as Good
Clinical Practice (GCP). These regulatory advancements have contributed to an
increase in the number of multinational companies conducting clinical trials in
India.
For instance, the COVID-19 pandemic saw
significant clinical trials in India, with companies like Bharat Biotech and
Serum Institute of India conducting large-scale trials for vaccines such as
Covaxin and Covishield. These trials not only helped in addressing the global
pandemic but also highlighted India’s critical role in the global clinical
trials landscape.
Key Market Challenges
Lack
of Skilled Workforce
One of the significant challenges facing the
bioanalytical testing services market in India is the shortage of a highly
skilled workforce. As the demand for bioanalytical testing services grows with
the expanding pharmaceutical and biotechnology sectors, there is a clear gap in
the availability of professionals who possess specialized skills in advanced
testing techniques and technologies. This lack of skilled labor directly
impacts the quality, efficiency, and scalability of testing services,
especially in a market that is rapidly evolving and requires adherence to
stringent global standards.
India's bioanalytical testing sector relies heavily on
professionals skilled in complex areas such as pharmacokinetics (PK),
bioavailability, and bioequivalence, among others. However, many testing
facilities in India struggle to attract and retain talent capable of performing
high-level analyses using state-of-the-art technologies like mass spectrometry,
liquid chromatography, and other cutting-edge instrumentation. While the Indian
pharmaceutical and clinical research industries have a large talent pool, the
highly specialized skills required in bioanalytical testing are often limited,
as bioanalytical testing is a niche area that requires specific academic and
practical training.
According to a report by NASSCOM, the Indian
pharmaceutical industry is expected to employ over 4.5 million people by 2025, but a
significant portion of these professionals will need further specialization in
bioanalytical testing to meet the increasing demands of drug development and
clinical trials. The gap in highly skilled talent is further compounded by the
rapid pace of technological advancements in the field, requiring professionals
to continuously upgrade their skills.
A survey by BioPharma Asia reported that over 60% of
bioanalytical service providers in India faced challenges in hiring
professionals with the necessary skills and experience in bioanalytical
testing, with 47% of respondents citing difficulty in finding qualified experts
in advanced testing techniques like HPLC and mass spectrometry. This shortage
of skilled professionals not only slows down the growth of the bioanalytical
services market but also increases labor costs as companies compete for the
limited talent pool available.
Key Market Trends
Growth
in the Contract Research Organization (CRO) Market in India
The Contract Research Organization (CRO) market in India is experiencing significant expansion, positioning the nation as a premier hub for pharmaceutical and biotech research. This growth is fueled by a compelling combination of cost-effective R&D services, a vast and diverse patient population, and a highly skilled scientific workforce. As global demand for efficient drug development escalates, India's CROs are increasingly sought after for outsourcing critical research services, from discovery to post-market surveillance. This trend solidifies India's strategic importance in the global pharmaceutical value chain and its role in accelerating medical innovation.
A key driver of this growth is the increasing trend of global pharmaceutical firms outsourcing their clinical trial services to India. The country's robust regulatory framework, which aligns with international Good Clinical Practice (GCP) standards, provides assurance of data quality and compliance. Furthermore, supportive government initiatives and streamlined approval processes have simplified the initiation and execution of clinical trials. This favorable environment reduces timelines and costs, making India a highly attractive destination for multinational companies looking to accelerate their drug development pipelines and gain a competitive advantage.
Indian CROs are rapidly integrating technological advancements to enhance service delivery and trial outcomes. The adoption of artificial intelligence (AI), machine learning, and advanced data analytics is revolutionizing clinical trial management. These digital tools streamline patient recruitment, enable real-time data monitoring, and improve the accuracy of clinical data analysis. By leveraging technology, Indian CROs offer more efficient, precise, and high-quality services in data management and clinical monitoring, boosting their competitive edge in the global market and ensuring superior trial performance for their clients.
The market is also benefiting from India's expanding capabilities in conducting complex, multi-phase clinical trials, including Phase I-IV studies. This expertise is particularly prominent in high-demand therapeutic areas like oncology, diabetes, and vaccines. Concurrently, the rising global demand for biosimilars and biologics has created new opportunities for specialized CRO services. Indian organizations are adept at navigating the specific regulatory pathways and testing requirements for these complex molecules, offering end-to-end support from development to post-market surveillance, further fueling market expansion..
Segmental Insights
Molecule
Insights
Based on Molecule, Small Molecule have
emerged as the fastest growing segment in the India Bioanalytical Testing
Service Market in 2024. Small molecules, which are typically
low-molecular-weight compounds, are central to the development of a wide range
of pharmaceutical products, particularly generic drugs, and are widely used in
the treatment of chronic diseases such as cancer, cardiovascular conditions,
and diabetes. The increasing prevalence of these diseases in India, coupled
with rising healthcare access, has driven the demand for small molecule-based
therapies. Additionally, small molecules are essential in the development of
cost-effective drugs, which is crucial for India, where there is a large market
for affordable pharmaceutical solutions.
The expansion of the Indian pharmaceutical sector,
particularly in the generic drug manufacturing industry, has also contributed
to the rise in small molecule testing services. India is one of the largest
suppliers of generic medicines globally, and the bioanalytical testing required
to ensure the efficacy and safety of these molecules is increasingly outsourced
to bioanalytical testing labs. Furthermore,
advancements in drug formulation technologies, along with the growing adoption
of biologics and biosimilars, have further highlighted the need for efficient
small molecule testing. The large number of clinical trials and regulatory
requirements to validate the quality of these molecules also plays a critical
role in the demand for bioanalytical testing services tailored to small
molecule drugs.
Test Insights
Based on Test, Pharmacokinetics (PK) have
emerged as the fastest growing segment in the India Bioanalytical Testing
Service Market during the forecast period. PK testing plays a crucial role in
evaluating the absorption, distribution, metabolism, and excretion (ADME) of a
drug, helping to understand its behavior within the body. As the demand for
both new drug development and generic drug approval continues to rise, PK
testing has become essential for ensuring the safety, efficacy, and proper
dosage of pharmaceutical products.
With an increase in clinical trials, particularly for
small molecules and biologics, PK testing is a vital step in the drug
development process to optimize drug formulations and ensure therapeutic
effectiveness. Regulatory authorities like the USFDA and CDSCO (Central Drugs
Standard Control Organization) require detailed PK studies to support drug
approvals, further driving the need for bioanalytical testing services in
India.
.png)
Download Free Sample Report
Regional Insights
Based on Region, South India have
emerged as the dominating region in the India Bioanalytical Testing Service
Market in 2024. South India has emerged as the dominating region in the India
Bioanalytical Testing Service Market in 2024 due to several key factors. The
region is home to a well-established and rapidly growing pharmaceutical and
biotechnology industry, which significantly contributes to the demand for
bioanalytical testing services. Cities like Hyderabad, Bangalore, and Chennai
have become major hubs for pharmaceutical research and development, with a high
concentration of contract research organizations (CROs), clinical research
institutions, and bioanalytical laboratories.
Hyderabad, often referred to as "India’s
Pharmaceutical Capital," houses a number of global pharmaceutical
companies, contract manufacturers, and biotech firms, further driving the need
for bioanalytical testing services. South India also benefits from a robust
infrastructure and favorable government policies aimed at fostering growth in
the life sciences sector, including initiatives like the establishment of
biotechnology parks and tax incentives for R&D activities. Additionally,
the region has a well-developed pool of skilled professionals in pharmaceutical
sciences and clinical research, making it an attractive destination for
bioanalytical testing service providers.
Recent Development
- In September 2024, the
Indian Council of Medical Research (ICMR) signed Memorandum of Agreements
(MoAs) with multiple industry and academic partners to advance first-in-human
Phase 1 clinical trials for four promising molecules. These collaborations
include the development of a small molecule targeting multiple myeloma in
partnership with Aurigene Oncology Limited, a Zika virus vaccine with Indian
Immunologicals Limited, a seasonal influenza vaccine with Mynvax Private
Limited, and the exploration of CAR-T cell therapy for chronic lymphocytic
leukemia with ImmunoACT. This initiative is designed to position India as a
leader in clinical development by improving its capacity for early-phase
clinical trials and fostering indigenous pharmaceutical innovations.
- In April 2024, India's biopharmaceutical
company Biocon embarked on developing its own version of Novo Nordisk's
weight-loss medication, Wegovy. The company aimed to introduce a
semaglutide-based product to tap into the burgeoning obesity treatment market,
projected to reach at least $100 billion by 2030.
- In February 2024, India achieved a
significant milestone by conducting its first human clinical trial of gene
therapy for haemophilia A at Christian Medical College (CMC) Vellore. This
pioneering study was supported by the Department of Biotechnology, the Centre
for Stem Cell Research (a unit of InStem Bengaluru), and Emory University, USA.
The innovative approach involved using a lentiviral vector to express a Factor
VIII (FVIII) transgene in the patient's own haematopoietic stem cells, enabling
the production of FVIII from specific differentiated blood cells.
- In October 2023, Roche Pharma India
launched its Clinical Trial Excellence project to enhance the capabilities of
public health institutions in conducting clinical trials and drug research. The
initiative aims to transform government hospitals into Centres of Excellence for
Clinical Research, elevating their position in the value chain. The Kalyan
Singh Super Specialty Cancer Institute (KSSSCI) in Lucknow, Uttar Pradesh,
became the first partner in this project. KSSSCI is a 750-bed state-of-the-art
cancer center involved in cancer research and education.
Key Market Players
- Syneos
Health Pvt Ltd
- Intertek
Group PLC
- SGS
SA
- IQVIA
Inc
- Icon
PLC
- Labcorp
Holdings Inc
- Charles
River Laboratories India Pvt. Ltd.
|
By Molecule
|
By Test
|
By Workflow
|
By Application
|
By End Use
|
By Region
|
- Small Molecule
- Large Molecule
- Others
|
- ADME
- PK
- PD
- Bioavailability, Bioequivalence
- Others
|
- Sample Preparation
- Sample Analysis
- Others
|
- Oncology
- Neurology
- Infectious Diseases
- Gastroenterology
- Cardiology
- Other
|
- Pharma & BioPharma Companies
- CDMO
- CRO
|
- East India
- West India
- North India
- South India
|
Report Scope
In this report, the India Bioanalytical Testing
Service Market has been segmented into the following categories, in addition to
the industry trends which have also been detailed below:
- India Bioanalytical Testing
Service Market, By
Molecule:
o Small Molecule
o Large Molecule
o Others
- India Bioanalytical Testing
Service Market, By
Test:
o ADME
o PK
o PD
o Bioavailability
o Bioequivalence
o Others
- India Bioanalytical Testing
Service Market, By
Workflow:
o Sample Preparation
o Sample Analysis
o Others
- India Bioanalytical Testing
Service Market, By
Application:
o Oncology
o Neurology
o Infectious Diseases
o Gastroenterology
o Cardiology
o Other
- India Bioanalytical Testing
Service Market, By
End Use:
o Pharma & BioPharma Companies
o CDMO
o CRO
- India Bioanalytical Testing
Service Market, By Region:
o East India
o West India
o North India
o South India
Competitive Landscape
Company Profiles: Detailed analysis of the major companies
present in the India Bioanalytical Testing Service Market.
Available Customizations:
India Bioanalytical Testing Service Market report
with the given market data, TechSci Research offers customizations according
to a company's specific needs. The following customization options are
available for the report:
Company Information
- Detailed analysis and
profiling of additional market players (up to five).
India Bioanalytical Testing Service Market is an
upcoming report to be released soon. If you wish an early delivery of this
report or want to confirm the date of release, please contact us at [email protected]